SOHMA is now offering Cold Laser Therapy, an FDA cleared medical device to help with pain and speed up the healing process.
There are a number of applications for Erchonia Corporation’s groundbreaking laser devices. Based on Erchonia’s Level one clinical study results in 2002, the FDA found it necessary to create a new regulatory category of medical devices: NHN Biostimulation lasers.

Check out all of the Erchonia Laser’s applications:
Chronic Low Back Pain
About 80 percent of adults experience low back pain at some point in their lifetimes. Of those, about 60% are treated with opioids, despite the opioid epidemic and minimum effectiveness (30%). Erchonia’s FX 635 laser showed a 72% success rate in their clinical trial, which received FDA market clearance in May 2018. The FX 635 treatment of chronic low back pain is groundbreaking in the pain management community and give patients a safer, more effective treatment option that will get them back on their feet in no time.
Body Contouring
Erchonia lasers are proven to target and release fat in areas that are difficult to target with regular diet and exercise. The Zerona laser is proven to target these difficult areas to open a transitory pore in the cell membrane to release fat. The Erchonia EML laser approved as an addition to traditional liposuction surgery. It is the first and only low-level laser to be given FDA market clearance for use immediately before liposuction. The painless laser treatment is applied prior to liposuction and liquefies the fat. This makes for a much easier removal of fat, and results in less post-op pain and bruising as well as a much shorter recovery time.
Neck and Shoulder Pain
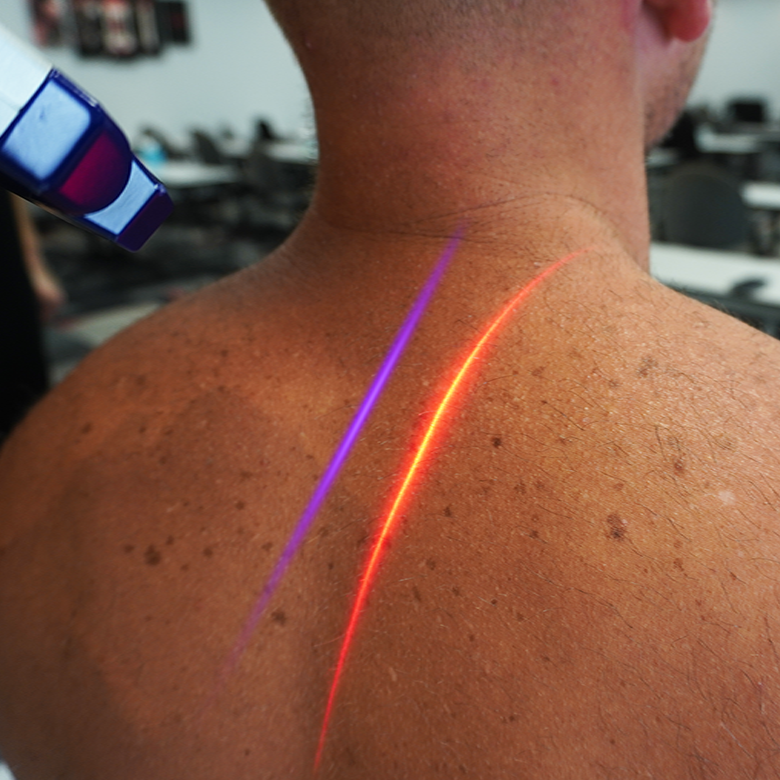
Erchonia low level lasers are proven to be effective in reducing and eliminating chronic pain in the neck & shoulders. Many patients find that this solution is a much more effective and immediate alternative to traditional pain medications. The Erchonia lasers have been proven through rigorous clinical trials in which subjects had to refrain from any therapies, beside the laser. However in practice doctors are combining with forms of chiropractors and therapy, to help patients lead a normal, active, and healthy life without debilitating pain.
Acne
Currently, there are many acne treatments available in the form of pills, lotions, tonics, chemicals, and more, but Erchonia has developed a new solution with the EVRL LASER. This FDA market cleared laser targets the actual type of bacteria that is responsible for causing acne, rather than targeting the skin. This treatment does not lead to irritated skin cells because it attacks the cause of acne at the root. Rather than spending many months or years on powerful antibiotics, creams, and more, patients can now attach their acne with painless low-level laser treatments. Cold lasers are currently undergoing studies for new indications for use. Though it is a relatively new field of medicine, Erchonia’s commitment to low-level laser treatment has made great advancements for laser technology. The future of medical lasers is certainly bright.
Obesity
Approximately two-thirds of U.S. adults are either overweight or obese and one-third are clinically obese. The Emerald laser is the only non-invasive device FDA Cleared to treat up to 40BMI. Published clinical data demonstrated over 70% of study individuals obtained 3 inch or greater circumference reduction following 6 weeks. During this time the individuals agreed to maintain their regular diet and exercise routine for the duration of the study, however healthy lifestyle changes are recommend.
Plantar Fasciitis
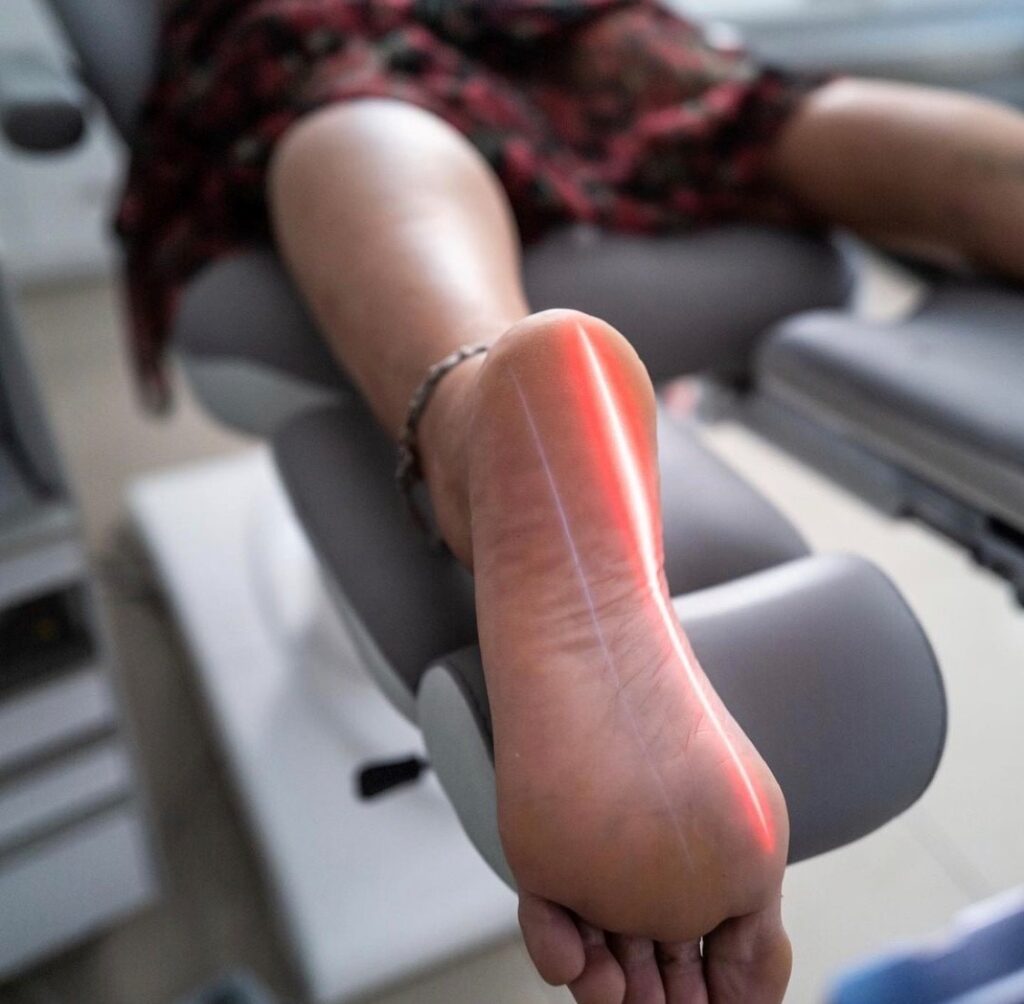
Plantar fasciitis is the most common cause of heel pain in adults, affecting 1 million persons each year in the United States. Treatment ranges from rest to surgical intervention. Erchonia’s LLLT has received FDA clearance as an adjunct treatment for Plantar Fasciitis due to the treatment’s safe and efficacious nature. In a 3 week study, on average individuals experienced a 50% reduction in pain, with pain reduction either improving or sustaining up to 12 months post treatment
Onychomycosis
Onychomycosis is an infection of the finger or toenails causing the unsightly appearance of nails. Current therapies include oral and topical medications, however medications do come with a risk of possible side effects. Another treatment option is Erchonia’s LLLT which received FDA Clearance in 2016. The treatment involves only 4 non-thermal laser sessions with no known side effects.
FAQ

General Knowledge
Q. What is low-level laser therapy (3LT®)?
A. Low-level laser therapy is the use of low intensity photonic energy as a treatment modality.
Q. How does Low-Level Laser (3LT®) work?
A. Photonic stimuli excite the body’s cells infusing them with energy, with the three primary reactions being, reduction of inflammation, cell function and increased blood flow.
Q. What is it used for?
A. The potential applications of low-level laser (3LT®) are almost limitless, however; to date Erchonia has received market clearance for Neck and Shoulder Pain, Breast Augmentation, Acne, Laser Assisted Liposuction, and Non-Invasive Body Contouring. Erchonia continues to conduct clinical trials on other applications.
Q. What are the benefits of low-laser therapy?
A. Low level laser therapy is a non-invasive, fast and effective modality that has been proven in clinical trials to reduce pain, reduce edema, and promote healing.
Q. How safe is low-level laser therapy (3LT®)?
A. Low level laser therapy has is very safe; the only general precaution is the use of special filtering glasses when a class 3B laser is in use.
Q. How deep into the tissue can laser light penetrate?
A. The depth of penetration is dependent on multiple factors including mass and density, however since low-level laser has been proven in clinical studies to effect subcutaneous cells; the point is low-level laser, does penetrate; as opposed to the heat lamp devices that do not.
Q. What the difference is between pulsed vs. constant wave?
A. Like their names imply, constant wave is a continuous emission of laser energy, without disruption, for the length of time the device is ON. Pulsed wave is controlled breaks in the wave, at predefined and programmed intervals.
Q. What is hertz as it relates to low-level lasers?
A. The predefined, controlled breaks in the laser emission, measured by the number of breaks per second, equals hertz.
Applications
Q. Is low-level laser therapy painful?
A. No, most people do not feel anything. For those that have reported a feeling, it is nothing more than a slight tingling.
Q. How is low-level laser therapy administered?
A. The mechanics of low low-level laser therapy is administered is based on the indication for use; however, the general process is the probe containing the laser diode(s) are held in place or moved gently over the treatment area at a distance of anywhere from 4” – 12.”
Q. How long does it take for the laser to heal or improve a condition?
A. This is dependent of the application; however, progress is immediately evident.
Q. What’s the difference between Lasers vs. LED?
A. Erchonia 3LT® devices used electric diodes, which are high end, culminated and strictly measured within a plus/minus .05%. LEDs are inexpensive, non-focused wide range light sources. The primary difference is in performance and depth of penetration. Laser diodes penetrate, working subcutaneously, LEDs do not affecting the surface only.
Research
Q. What clinical studies have been conducted using low-level laser?
A. Erchonia has sponsored numerous clinical trials and continues to promote low-level laser as a modality through ongoing research. To see the completed study results and the on-going study progress, click RESEARCH
Compliance
Q. Is low-level laser therapy FDA market cleared?
A. The FDA clears for market devices and specific indications for use, this is sometime referred to by persons outside the FDA as “FDA Approval”, although it is a term unacceptable to the FDA. All Erchonia devices have received a FDA market clearance or were self-certified in accordance to FDA regulation.
Q. Are Erchonia low-level laser devices tested to ensure safety?
A. Erchonia Corporation develops, designs, and manufactures devices in accordance to both FDA and International Standards for Medical Device Quality Standards. Prior to release to production, finished devices are tested to Medical Safety Standards for Laser Controls, EMC, and Safety.
Contraindications
Q. Are there any conditions which would prevent me from low-level laser treatment?
A. There are no code regulated contraindications; however, since there are no long term evaluations on certain conditions, Erchonia does not recommend use on pregnant women or persons with a pace maker.
Q. Does low-level laser therapy cause heat damage or cancer in the tissue?
A. No, low-level laser by virtue of design is non-heat producing and does not alter the cell structure. The laser irradiation is non-ionizing, meaning it does not collect in the tissue.
Q. Are there any side effects?
A. Some persons have reported a sense of deep relaxation that may cause drowsiness
Q. Are there any complications to the treatments?
A. There are no known and / or published adverse effects of low-level laser therapy.